Comparison of the knowledge, attitudes, and perception of barriers regarding adverse drug reaction reporting between pharmacy and medical students in Pakistan
Article information
Abstract
Purpose:
The goal of this study was to compare the knowledge and attitudes of pharmacy and medical students regarding adverse drug reactions (ADRs), as well as their perceptions of barriers to ADR reporting, in a Higher Education Commission-recognised Pakistani university.
Methods:
A cross-sectional study was conducted among final-year pharmacy (n=91) and medical (n=108) students in Pakistan from June 1 to July 31, 2014. A self-administered questionnaire was used to collect the data. The responses of pharmacy students were compared to those of medical students.
Results:
Pharmacy students had a significantly better knowledge of ADRs than medical students (mean±SD, 5.61±1.78 vs. 3.23±1.60; P<0.001). Gender showed a significant relationship to knowledge about ADRs, and male participants were apparently more knowledgeable than their female counterparts (P<0.001). The attitudes of pharmacy students regarding their capability to handle and report ADRs were significantly more positive than those of medical students (P<0.05). In comparison to pharmacy students, a lack of knowledge of where and how to report ADRs was the main barrier that medical students perceived to ADR reporting (P=0.001).
Conclusion:
Final-year pharmacy students exhibited more knowledge about ADRs and showed more positive attitudes regarding their capacity to handle and report ADRs than final-year medical students.
INTRODUCTION
The reporting of adverse drug reactions (ADRs) is still often ignored by healthcare professionals in Pakistan. Researchers have found that the high prevalence of substandard medicines, the irrational use of drugs, medication errors, and drug-related morbidity and mortality highlight the need for pharmacovigilance centres in Pakistan [1]. The under-reporting of ADRs in Pakistan may lead to further complications associated with the above-mentioned problems. Furthermore, healthcare professionals in Pakistan also need to be educated about the importance of ADR reporting. It is imperative for future pharmacists and physicians to be well trained, knowledgeable, and aware of how to identify, prevent, manage, and report ADRs. To our knowledge, only one study of this issue have been conducted in Pakistan, and they have focused on comparing the knowledge and attitudes of medical and dental students in Pakistan towards ADRs [2].This study aimed to compare the knowledge and attitudes of medical and pharmacy students in Karachi, Pakistan regarding ADRs and ADR reporting.
METHODS
A descriptive, cross-sectional study was conducted among final-year pharmacy and medical students of a Higher Education Commission-recognised Pakistani university from June 1 to July 31, 2014. The sampling frame included the final-year students of both professional programs, and each participant was approached individually about participating in this study. A self-administered questionnaire was used to collect data from the participants. The questionnaire was distributed to the students by two of the authors, who were responsible for data collection. The same authors were also assigned the responsibility of explaining the questionnaire to the students. The questionnaire was also subjected to content validity and reliability testing. An initial draft was designed by the authors after an extensive literature review [2-5], after which it was sent to four experts from the Faculty of Pharmacy and the Faculty of Medicine. Expert opinions were provided with the goal of making the questionnaire more relevant and significant. A pilot study was also conducted on a small sample of both pharmacy and medical students (n=10). The responses from these participants were not included in the final analysis. Changes were made to the questionnaire with the aim of making it more brief and simple. The internal consistency of the questionnaire was measured with the reliability coefficient, Cronbach’s alpha, which was found to be 0.81.
The finalized questionnaire was divided into six sections. The first section included demographic information, such as gender, degree program, and previous experience with and/or exposure to ADRs. The second section included questions assessing the knowledge of students regarding ADRs and ADR reporting. This section comprised 10 multiple-choice questions. One point was given for each correct answer and zero points were given for each incorrect answer. The maximum possible score was 10 and the minimum possible score was 0. A score <7 was considered to indicate a poor level of knowledge, and a score ≥7 was considered to indicate a good level of knowledge.
The third section assessed the attitudes of pharmacy and medical students towards ADRs and ADR reporting. This section included eight questions, responses to which were measured on a four-point Likert scale. A score of 1 indicated strong agreement, a score of 2 indicated agreement, a score of 3 indicated disagreement, and a score of 4 indicated strong disagreement. A mean score ≤2 was considered to reflect a positive overall attitude, while a score >2 was considered to indicate a negative overall attitude. The fourth section of the questionnaire examined students’ perceptions of reasons for not reporting ADRs. Students were asked to select the likely reasons for not reporting ADRs, and multiple selections were possible. The fifth and sixth sections of the questionnaire assessed participants’ sources of information about ADRs and the reasons for ADR reporting, respectively.
The sample included all the final-year PharmD and medical students who were enrolled as full-time students in the Higher Education Council-recognised university in which the study was carried out. A list of all the enrolled students was obtained from the lecturers of both the Faculty of Medicine and the Faculty of Pharmacy. Questionnaires were distributed to the participants in face-to-face meetings. Permission was obtained from the relevant lecturers and preceptors of both faculties. Students were provided with a comprehensive explanation of the objectives of this study. Students were informed that completing and returning the questionnaire would be considered to indicate their consent to participate in this study. The data were handled with a high level of anonymity and confidentiality.
The statistical analysis of the data was carried out using IBM SPSS ver. 20.0 (IBM Co., Armonk, NY, USA). Descriptive analysis was applied to present the results in percentages. The mean and SD were also computed for the variables in the study. The Kolmogorov-Smirnov test was performed to assess the nature of the data distribution. Since the data were normally distributed, the independent t-test was applied to compare the mean score of the section that assessed students’ knowledge of ADRs with their demographic information. The chi-square test was applied to identify associations between dependent and independent variables. However, if 25% or more of the cells in a given table had an expected frequency of <5, Fisher’s exact test was performed instead of the chi-square test. The Monte Carlo test (two-sided) with a 99% confidence level was also used to estimate the Fisher’s exact P-value, and P-values <0.05 were considered to indicate statistical significance.
RESULTS
A total of 91 pharmacy students and 108 medical students completed the questionnaire, yielding a response rate of 94.6%. The mean ages of the pharmacy and medical students were 23.78±0.72 years and 24.14±0.64 years, respectively. A significant gender difference (P<0.001) was found in the mean knowledge score of the participants, with male participants (n=84) showing more knowledge of ADR reporting (4.98±2.02) than their female counterparts (n=115, 3.83±1.95). Another important finding of this study was a significant difference (P<0.001) between pharmacy and medical students in knowledge about ADR reporting, with pharmacy students showing a greater degree of knowledge (5.61±1.78) about ADR reporting than medical students (3.23±1.60). Although a significant difference was observed between the pharmacy and medical students, the mean knowledge score of both groups was inadequate. Similarly, a difference was observed between the mean knowledge scores of respondents who reported previous exposure to ADRs (4.54±2.04) and their counterparts (4.02±1.85); however, this difference was not statistically significant (P= 0.156). The complete set of relationships between demographic variables and respondents’ knowledge of ADRs is presented in Table 1.

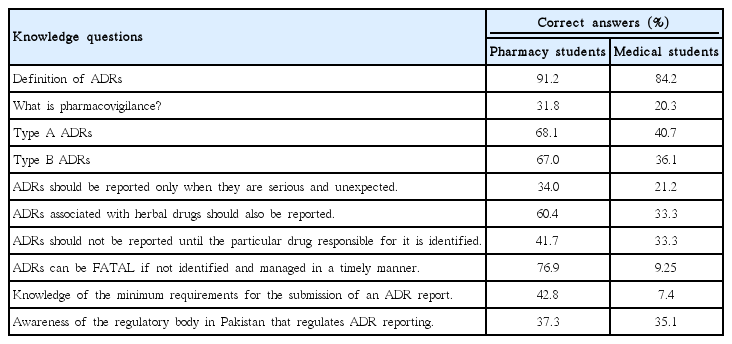
Relationships of the demographic characteristics of the participants with their knowledge scores (N=199)
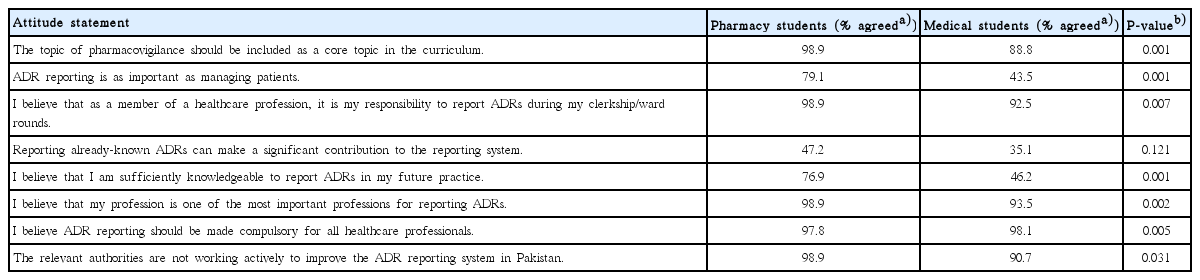
Our results showed that pharmacy students exhibited more knowledge regarding every aspect of ADRs and their reporting than medical students. However, the discrepancy between pharmacy and medical students varied from question to question. When asked if ADRs can be fatal if not identified and managed in a timely manner, 76.9% of pharmacy students answered correctly, compared to only 9.25% of medical students. Similarly, the knowledge gap between the two groups was wide regarding their knowledge of the minimum requirements for the submission of ADRs (pharmacy, 42.8%; medical, 7.4%). Conversely, the knowledge level of pharmacy and medical students was almost the same regarding awareness of the regulatory body in Pakistan that regulates ADR reporting (37.3% and 35.1%, respectively). Both groups showed a similar ability to identify the correct definition of ADRs (91.2% and 84.2%, respectively). Although participants showed an adequate overall knowledge of ADRs, surprisingly, only a few participants in either group were aware of the term ‘pharmacovigilance’ (31.8% and 20.3%, respectively). The responses to all of the questions assessing students’ knowledge of ADRs are presented in Table 2. Our results showed that the overall attitude of pharmacy students towards these issues was positive (mean score±SD, 1.82±0.2), in contrast to medical students (mean score±SD, 2.1±0.32). Pharmacy students reported more positive responses to all of the attitude statements than the medical students (P<0.05 for almost all of the statements related to attitude). However, while pharmacy students overwhelmingly agreed that the reporting of already-known ADRs makes a significant contribution to the reporting system, their responses did not differ from those of the medical students to a statistically significant extent (P=0.121). The responses of pharmacy and medical students towards attitude statements are summarized in Table 3.
Proportions of final-year pharmacy and medical students who correctly answered knowledge questions regarding ADRs and ADR reporting
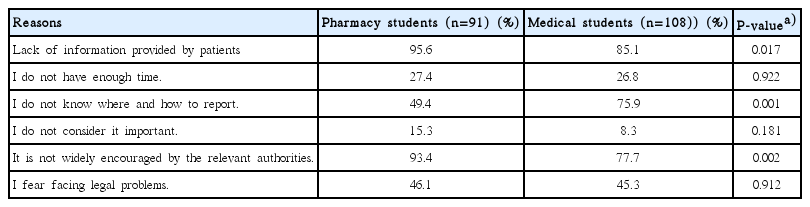
The majority of the students believed that the absence of information provided by the patient was the main reason for not reporting ADRs; however, more pharmacy students (95.6%) agreed with this statement than medical students (85.1%). This difference was also statistically significant (P<0.05). Similarly, both groups reported that a lack of encouragement on the part of the authorities was a reason for non-reporting, although more pharmacy students agreed with this statement than medical students (93.4% vs. 77.7%, P=0.002). Interestingly, 75.9% of medical students answered that a lack of knowledge about where and how to report ADRs is a major reason for non-reporting, although only 49.4% of pharmacy students reported this reason (P=0.001). The reasons cited by the participants for not reporting ADRs are tabularized in Table 4.
The main source of information used by pharmacy students was the Internet (60%) while medical students relied mainly on textbooks (47%). Detailed information about this is presented in Fig. 1. Similarly, Fig. 2 illustrates the purposes for reporting ADRs cited by pharmacy and medical students; the majority of pharmacy and medical students considered the purposes of ADR reporting to be to identify drugs involved in ADRs, to calculate the incidence of ADRs, to share information, to identify unrecognized ADRs, and to improve patient safety.
DISCUSSION
The results indicating that both pharmacy and medical students demonstrated an inadequate level of knowledge about ADRs may be explained by the lack of courses on ADRs and pharmacovigilance in their curricula. In this study, male students were found to be more knowledgeable than female students. However, this result does not correspond to that of another study conducted in Malaysia, in which a superior level knowledge was reported among female students [4]. This discrepancy could be explained by cultural differences, as male students in Pakistan are more practical and career-oriented, and are therefore more likely to be involved in various types of hospital internships, workshops, and seminars that would allow them to become acquainted with issues such as ADRs. It was also noteworthy that no significant difference was observed between the mean knowledge scores of students with previous experience of/exposure to ADRs and those who had no such background. This might indicate a systematic flaw in this aspect of the educational/training system, suggesting that it is not able to groom students who will be able to make significant contributions in the area of ADR reporting.
The respondents’ lack of understanding about pharmacovigilance, which was observed among both pharmacy and medical students, needs to be addressed. Previous studies on this topic have reported similar results [6]. Another remarkable result was the lack of knowledge in both groups about the existence of a drug regulatory authority for reporting ADRs. The findings of this study suggest that significant differences exist in the knowledge of pharmacy and medical students as assessed by a range of knowledge questions. This discrepancy might exist because pharmacy students take two to four semesters of pharmacology and two to four semesters of clinical pharmacy in their PharmD curriculum. In contrast, medical students study pharmacology for only one to two semesters in their medical curriculum. This indicates that PharmD students undergo rigorous training in pharmacology and clinical pharmacy, which is likely to be the reason that they displayed more knowledge than medical students in this domain. Courses on pharmacovigilance must be included in the curricula of both pharmacy and medical students in order to improve their knowledge thereof. Similarly, clinical sessions and clinical/research projects should also be implemented, and the monitoring of ADRs should be considered to be an integral part of patient care. These recommendations are also backed by the Uppsala Monitoring Centre, the international collaborating centre for ADR monitoring. According to the Uppsala Monitoring Centre, pharmacovigilance courses should be taught at the undergraduate level to healthcare professional students alongside the rational use of medications [7]. The attitude of pharmacy students towards these issues was positive, in contrast to medical students. This result agrees with the results of previous studies that have likewise found that pharmacists have a positive attitude towards their ability to handle and report ADRs [8]. Most students from both professional programs agreed that the topic of pharmacovigilance should be included as a core subject. This shows the positive attitude of the students towards the importance of pharmacovigilance, which they consider to be a weak link in their curricula. However, the data suggest that pharmacy students were more positively inclined towards recognizing the importance of pharmacovigilance than medical students. This discrepancy is problematic; medical students require more rigorous knowledge and training in ADRs and pharmacovigilance, as they are healthcare professionals of the future who will bear a great responsibility for patient care.
The current study also explored the reasons given by pharmacy and medical students for not reporting ADRs. The absence of information provided by the patient and a lack of encouragement from the authorities were the major reasons reported in this study. These results, however, differ from those of several other studies, which concluded that a lack of awareness of how and where to report ADRs were the major reasons for the underreporting of ADRs [9]. This inconsistency is likely due to patients’ distrust of pharmacists, which discourages them from sharing their health-related problems [10]. Another issue could be related to the inadequate functioning of the drug regulatory authority in Pakistan. Overall, our results demonstrate the need for raising awareness about ADR reporting in order to strengthen the bridge between the regulatory authorities and healthcare facilities in Pakistan.
The major strength of this study is that it focused on an issue that has not been adequately studied. The comparative nature of this study differentiates it from other published studies on pharmacovigilance in Pakistan. The questionnaire was subjected to content validation and reliability testing before distribution to the participants. This could be considered an additional strength of this study, as it may enhance the readers’ confidence in the results. However, the results must be interpreted with caution, as the results of a single-centre study may not be generalizable to the entire population. Despite this limitation, we believe that our results are a valuable contribution to the existing literature in light of the scarcity of relevant data. Furthermore, our results encourage researchers to investigate this issue by assessing the situation in different pharmacy and medical schools in Pakistan.
In conclusion, it is suggested that although pharmacy students have more knowledge about ADRs and a more positive attitude towards ADR reporting and pharmacovigilance than medical students, students in both professional programs still lack a basic understanding of these concepts. It is imperative that ADR reporting and pharmacovigilance be incorporated as core subjects in the curricula of future healthcare professionals, in order to best address the needs of the patients.
Notes
No potential conflict of interest relevant to this article was reported.
SUPPLEMENTARY MATERIAL
Audio recording of the abstract.